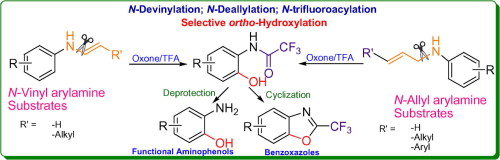
Direct C–N bond cleavage of N-vinyl or N-allyl arylamines: a metal-free strategy for N-devinylation and N-deallylation - Tetrahedron Lett. - X-MOL

Catalysts | Free Full-Text | PdI2-Based Catalysis for Carbonylation Reactions: A Personal Account | HTML

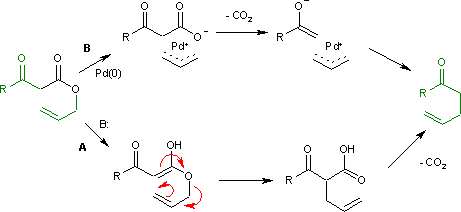
Hydrolytic Deallylation of N‐Allyl Amides Catalyzed by PdII Complexes - Ohmura - 2008 - European Journal of Organic Chemistry - Wiley Online Library
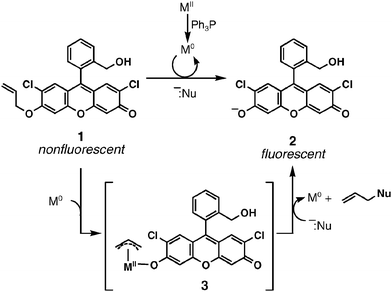
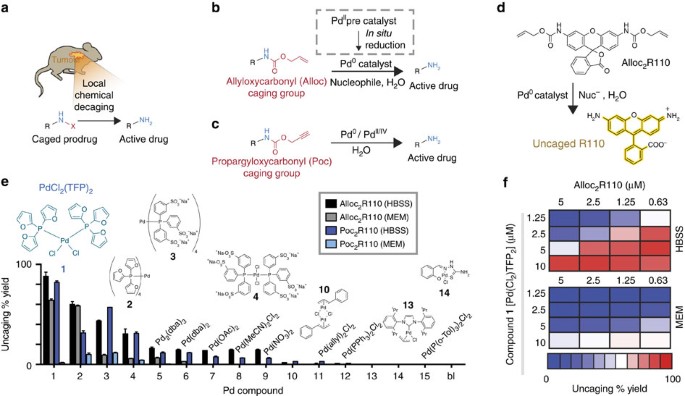
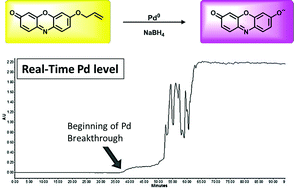
Studies of a fluorogenic probe for palladium and platinum leading to a palladium-specific detection method - Chemical Communications (RSC Publishing)

Hydrolytic Deallylation of N‐Allyl Amides Catalyzed by PdII Complexes - Ohmura - 2008 - European Journal of Organic Chemistry - Wiley Online Library

N-allyl-N-sulfonyl ynamides as synthetic precursors to amidines and vinylogous amidines. An unexpected N-to-C 1,3-sulfonyl shift in nitrile synthesis. - Abstract - Europe PMC

Facile and selective cleavage of allyl ethers, amines and esters using polymethylhydrosiloxane–ZnCl2/Pd(PPh3)4 - ScienceDirect
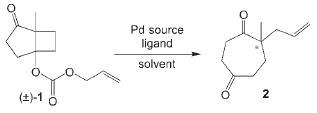
Palladium-Catalyzed Synthesis of Substituted Cycloheptane-1,4-diones by an Asymmetric Ring-Expanding Allylation (AREA)
![Elaboration of the ether cleaving ability and selectivity of the classical Pearlman's catalyst [Pd(OH)2/C]: concise synthesis of a precursor for a myo-inositol pyrophosphate - ScienceDirect Elaboration of the ether cleaving ability and selectivity of the classical Pearlman's catalyst [Pd(OH)2/C]: concise synthesis of a precursor for a myo-inositol pyrophosphate - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0040402012013944-sc6.jpg)
Elaboration of the ether cleaving ability and selectivity of the classical Pearlman's catalyst [Pd(OH)2/C]: concise synthesis of a precursor for a myo-inositol pyrophosphate - ScienceDirect

N-allyl-N-sulfonyl ynamides as synthetic precursors to amidines and vinylogous amidines. An unexpected N-to-C 1,3-sulfonyl shift in nitrile synthesis. - Abstract - Europe PMC

Overcoming the Deallylation Problem: Palladium(II)-Catalyzed Chemo-, Regio-, and Stereoselective Allylic Oxidation of Aryl Allyl Ether, Amine, and Amino Acids.,Organic Letters - X-MOL
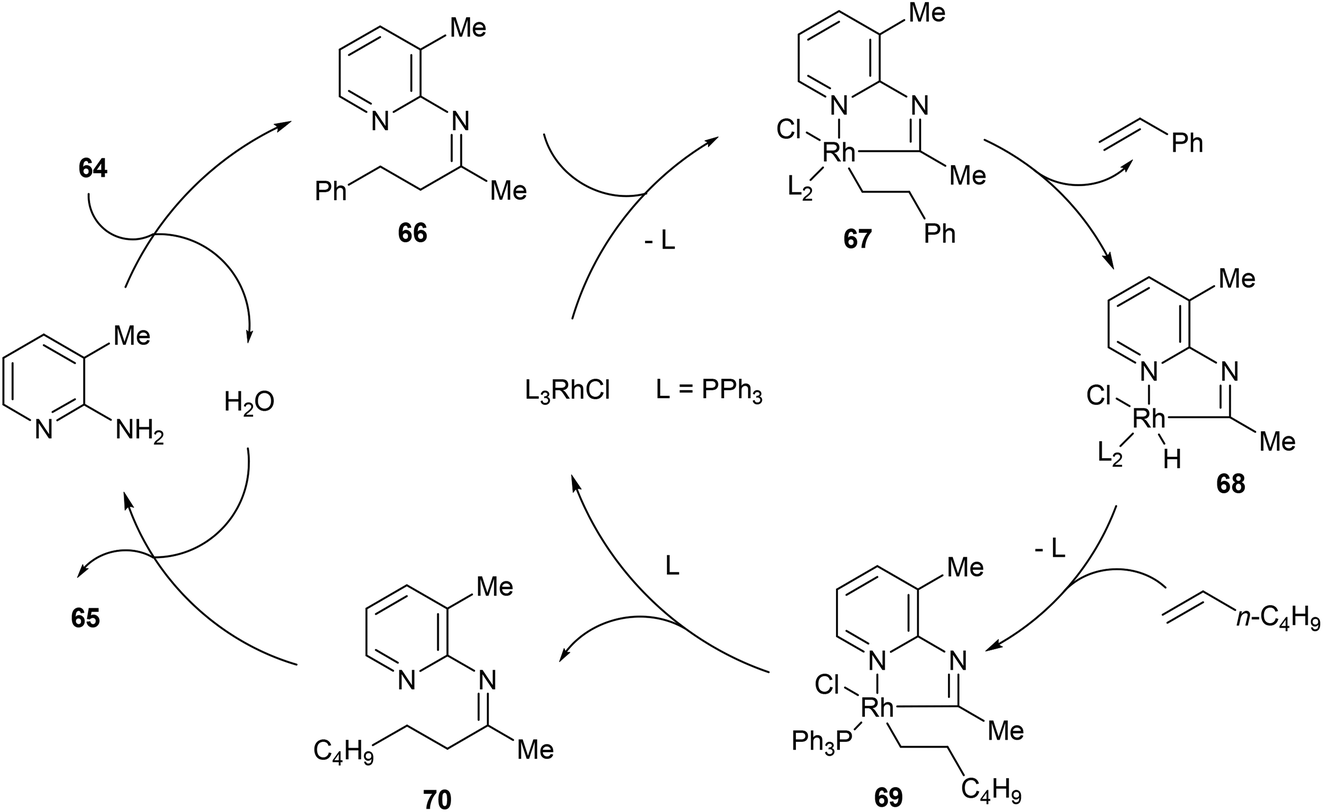
Direct activation of relatively unstrained carbon–carbon bonds in homogeneous systems - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C4QO00053F
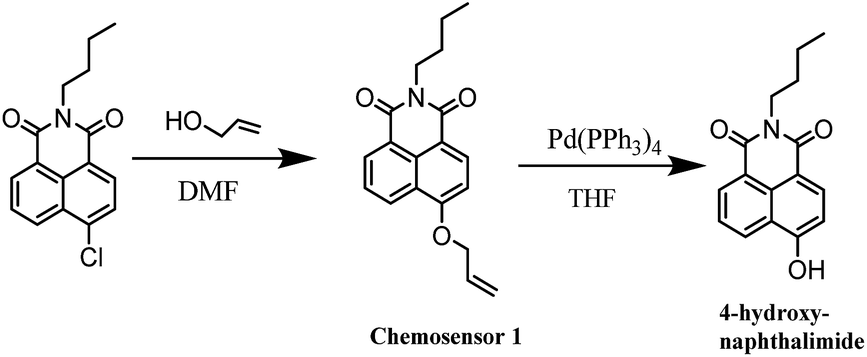
Triphenylphosphine-assisted highly sensitive fluorescent chemosensor for ratiometric detection of palladium in solution and living cells - RSC Advances (RSC Publishing)

Fullerene-bearing porous polymer via ball-milling approach and its palladium composite for catalytic deallylation - ScienceDirect

Palladium‐Catalyzed Oxidative Sulfamidation: A Stereoselective Synthesis for Enesulfonamides - Panda - 2017 - Advanced Synthesis & Catalysis - Wiley Online Library